“The negative pressure helmet is an incredibly innovative device which will immediately fill a significant and urgent need. This novel device will greatly benefit patients with COVID-19 and other aerosol-transmitted diseases, and will also aid in protection of health care workers by preventing disease transmission.”
Significant Need
Resource management and control of transmission are two of the greatest challenges faced by providers fighting the COVID-19 pandemic. Depending on the progression and severity of the disease, many patients must be placed in negative pressure rooms and/or on mechanical ventilators, which are in limited supply and pose an elevated risk to healthcare workers due to potential aerosolization of the virus. The number of hospitalized patients in need of breathing assistance has exploded since the pandemic began, leaving hospitals overtaxed, ventilators in high demand, and doctors forced to make life and death decisions about who receives ventilators and who does not.
Solution
University of Michigan researchers and engineers have developed a solution that provides negative pressure and PPE all in one. The helmet (called Aerosolve) enables the isolated use of a Heated High Flow Nasal Cannula (HFNC) or nebulized medications, sparing the need for a mechanical ventilator or potentially allowing earlier transition from mechanical ventilation.
Aerosolve’s compact design effectively creates a personal negative pressure environment wherever the patient is, and it maintains that environment even if the patient requires movement (imaging, testing, bathroom, etc.).
Competitive Advantage
Rapid setup and readily scalable
Allows clinicians to use all the tools in their medical arsenal without fear of contracting the illness themselves
Spares Personal Protective Equipment (PPE)
Comfortable for the patient
Beneficial in isolated environments (ground and air ambulances, cruise ships, aircraft carriers, etc.)
Value beyond pandemics – useful in other respiratory conditions where risk of infectious transmissibility is high
Endorsements
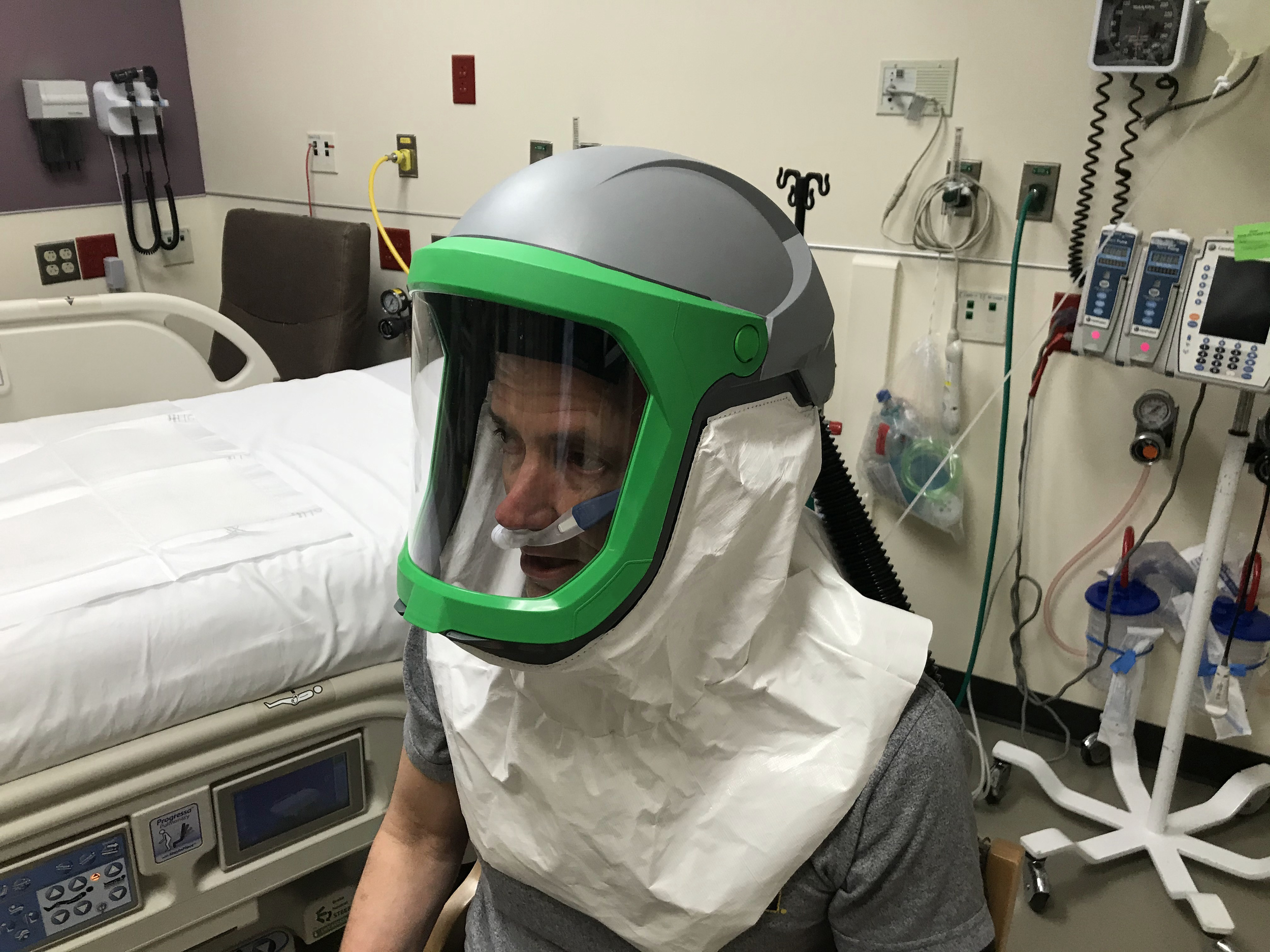

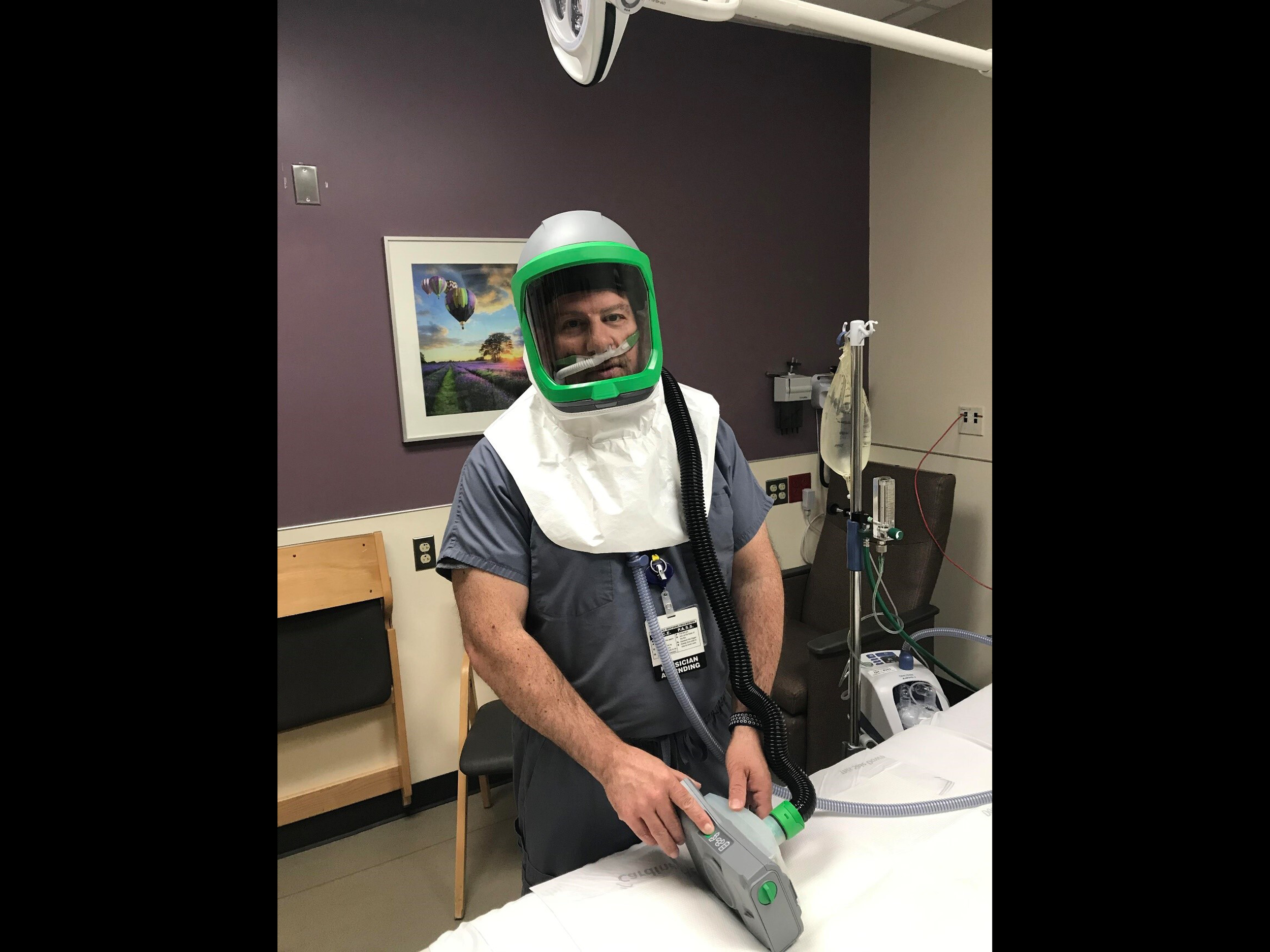
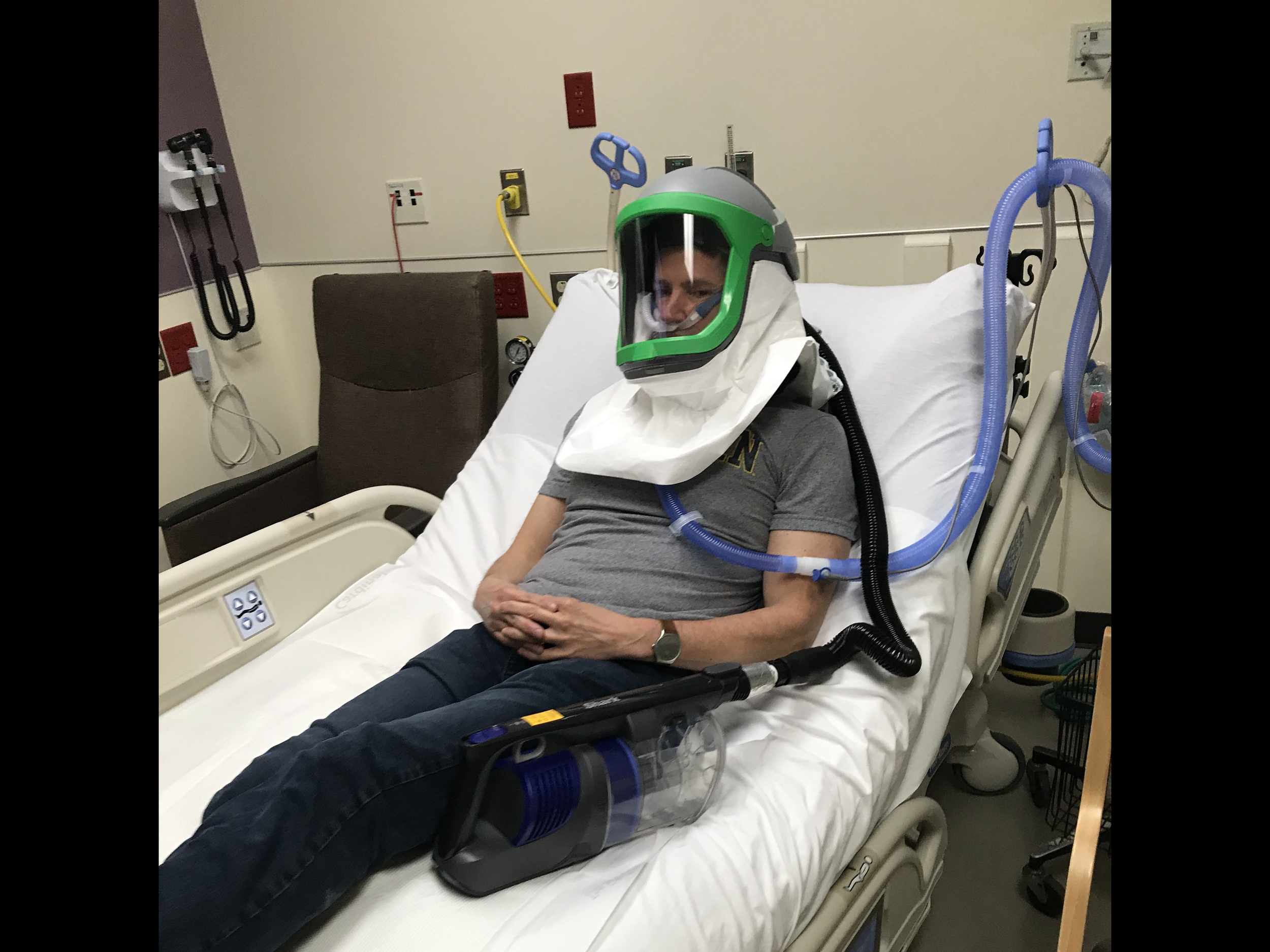
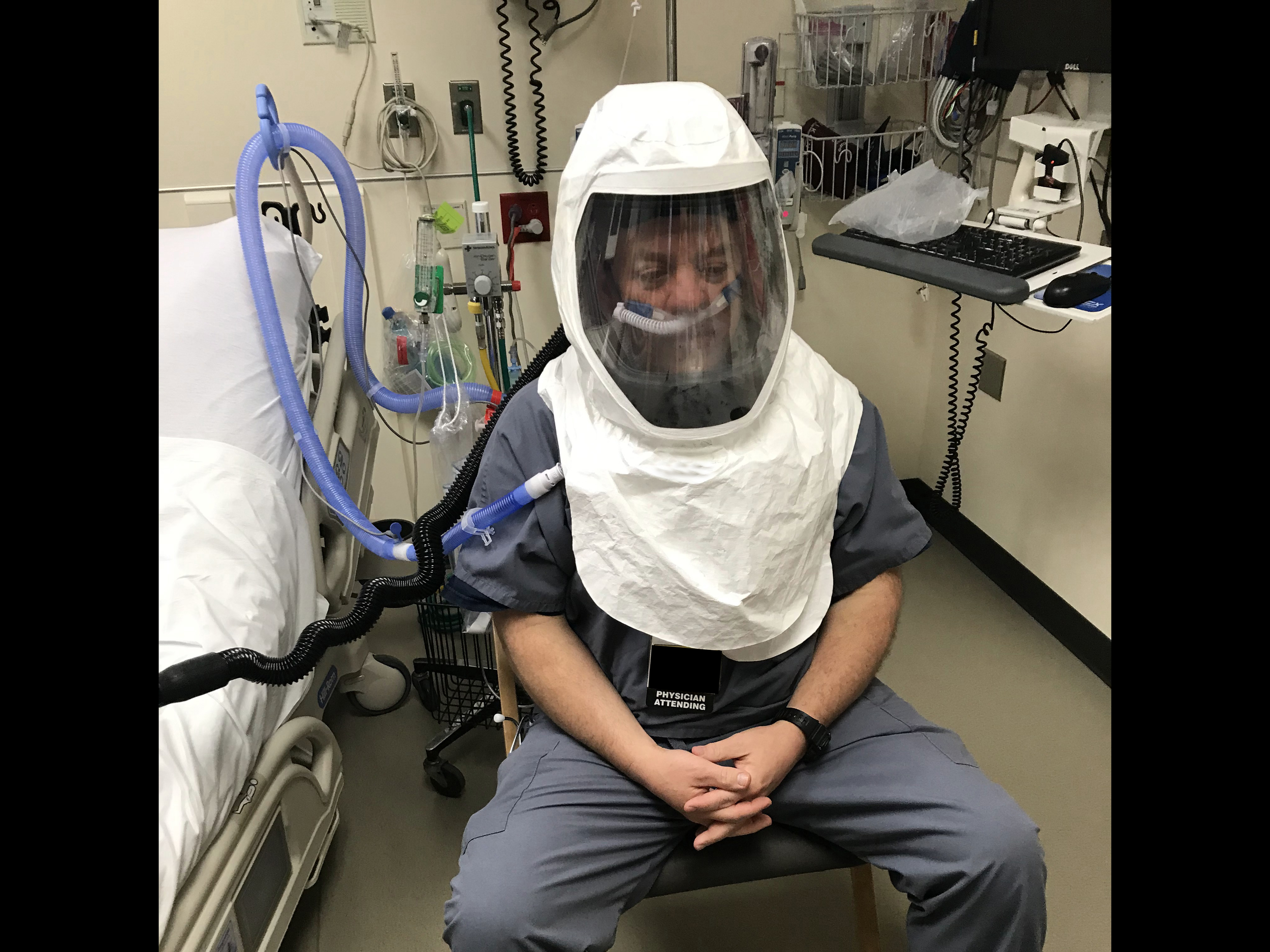
Fog Testing Demonstrations
Fog introduced at flows greater than HHFNC. Applied vacuums demonstrate no fog leakage, even with open face shield.
Commercialization Path
The team has filed a provisional patent. Quick FDA approval is anticipated as the HHFNC component is already in the marketplace. The device could conceivably go to market with a delivery of at least 1000 units. Production could ramp up to 100,000 units in the quarters that follow.
If you are interested in supporting work on this project, contact the Max Harry Weil Institute for Critical Care Research and Innovation.
Aerosolve in Action
Aerosolve in the Media
Disclosures
FlexSys, Inc. is a startup from the University of Michigan. Dr. Sridhar Kota holds equity in FlexSys, Inc.



















